



Despite a landscape clouded in complexity,
established and emerging biomarkers are expanding
our view of patient populations, and biomarker
testing could provide a more comprehensive patient
profile and lead to more informed decisions.


This list of gastric cancer biomarkers is not exhaustive. Other biomarkers may exist that are not mentioned on this website.
CLDN18.2=Claudin 18.2; FGFR2b=fibroblast growth factor receptor 2b; HER2=human epidermal growth factor receptor 2; MSI=microsatellite instability; PD-L1=programmed death-ligand 1.
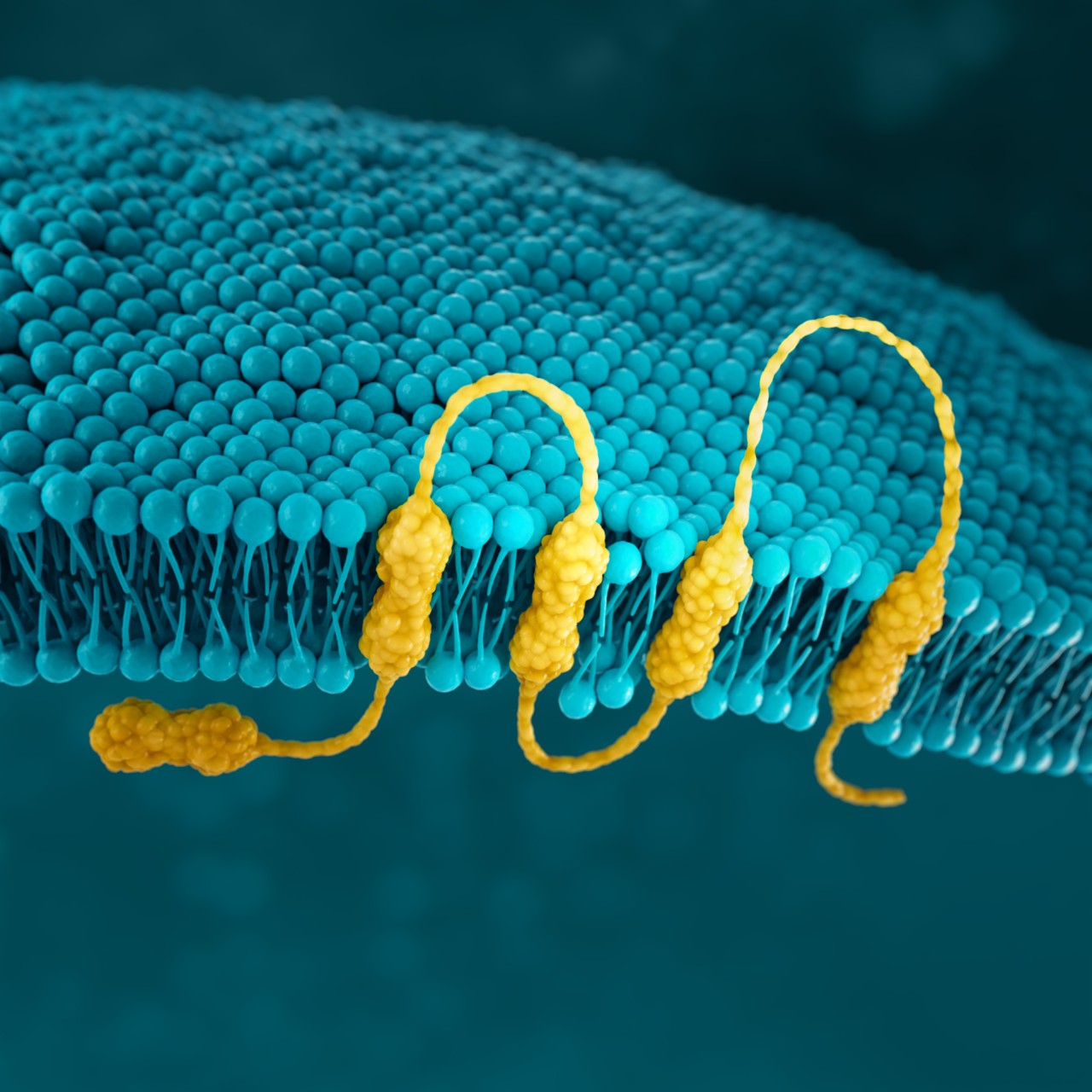
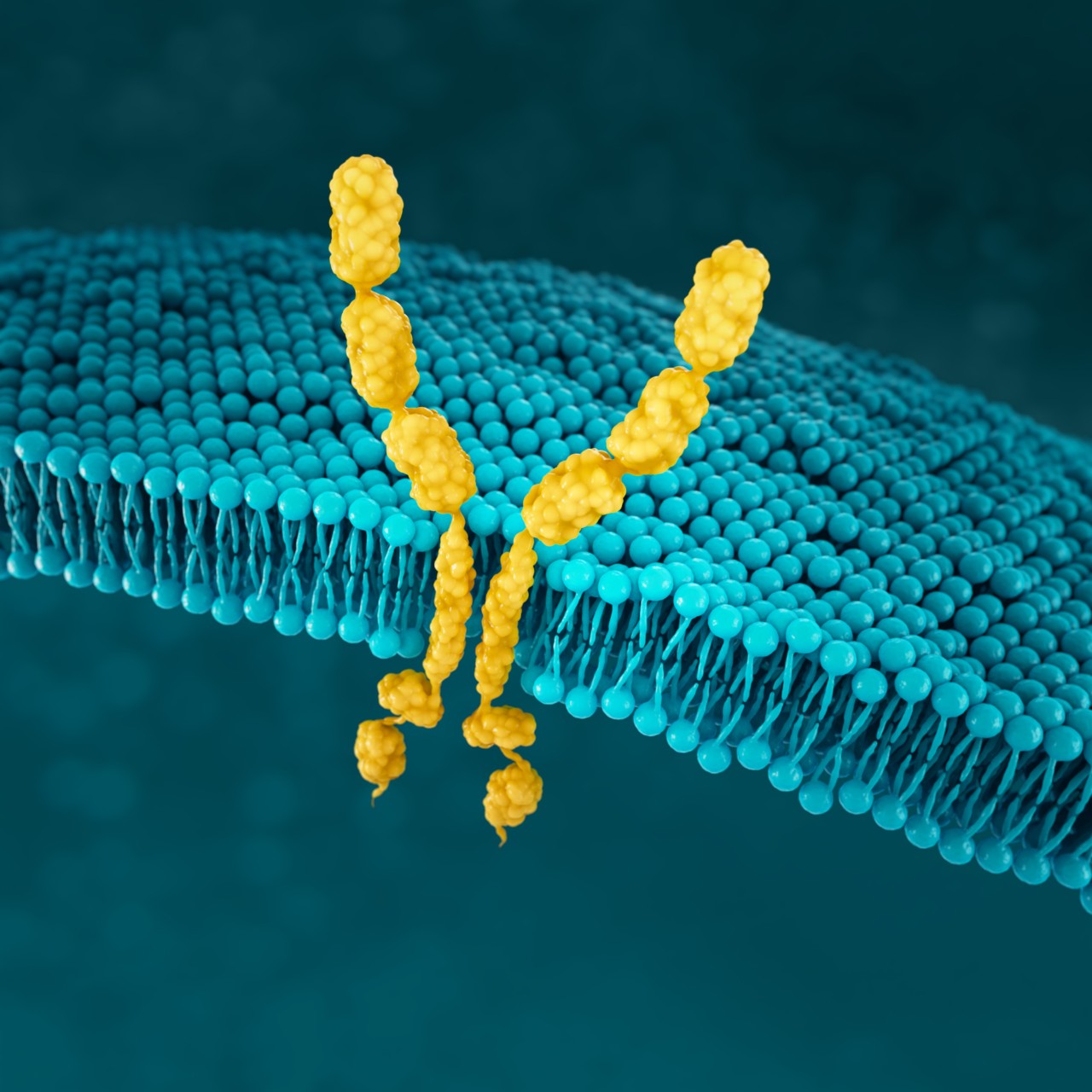
CLDN18.2
Claudins are a family of transmembrane proteins:5,34
Claudins are a major component of epithelial and endothelial tight junctions, which are involved in controlling the flow of molecules between cells.5-7
Claudins are present throughout the body, but two specific splicing isoforms of CLDN18 are localised to certain tissue types:5,34
Preclinical studies have shown that CLDN18.2 may become more exposed and accessible to antibodies as gastric tumours develop.5,38,39
CONFINED IN HEALTHY TISSUE
In gastric epithelial cells, CLDN18.2 is typically buried in the tight junction supramolecular complex.5,7,39
It functions to regulate selective barrier properties and contributes to cell-to-cell
epithelial adhesion.5–8
EXPOSED IN TUMOURIGENESIS
Malignant transformation leads to polarity disruptions and structure loss.38,39 As a result, CLDN18.2 may be more exposed and accessible to antibodies.5,38,39
RETAINED DURING TRANSFORMATION
The presence of CLDN18.2 is retained throughout malignant transformation, both in the primary tumour site and metastatic disease.5,38
Although not present in healthy tissues beyond gastric epithelial cells, CLDN18.2 may become activated in oesophageal, pancreatic, lung, and ovarian tumours as well.5
While approximately 70% of locally advanced and mG/GEJ cancers express CLDN18.2 (at any level), recent studies have shown approximately 33% of mG/GEJ patients are CLDN18.2 positive (high expression).21


Few patients with locally advanced or mG/GEJ cancer who are CLDN18.2+ (high expression*) also test positive for other biomarkers.21
When evaluating the relationship between CLDN18.2 and other biomarkers, current data suggest there is limited overlap.21








* High expression levels of CLDN18.2: 2+ and 3+ intensity in ≥75% tumour cells.
† Study population was limited to 350 Caucasian patients with mG/GEJ cancer, of which 117 patients had high expression of CLDN18.2. FGFR2b was not evaluated in this study.22
CLDN18.2 is expressed in both diffuse-type tumours and intestinal-type tumours.7
FGFR2b
FGFR2b is a receptor tyrosine kinase that has a role in normal cell development42
NORMAL CELL

In normal cells, FGFR signalling is an essential component for cell development. Deregulated FGFR2 pathway, through mutations or translocations, plays a critical role in several tumour types.46
GASTRIC CANCER CELL

FGFR2 overexpression and up-regulated signalling may be key events in a subtype of gastric cancer. Detection of FGFR2 amplification has been the mainstay for pre-screening patients for FGFR2 receptor overexpression.46
FGFR2b is an emerging biomarker that introduces another way to identify patients with mG/GEJ cancer10
FGFR2b positivity can be observed in 30% of mG/GEJ cancers.22
FGFR2b positivity: overexpression (IHC) and/or gene amplification by ctDNA (NGS).
Detecting FGFR2b can be done with the following tests:15,16
| Biomarker | Biomarker | Prevalence overlap | Reference |
|---|---|---|---|
| FGFR2b | CLDN18.2 | Unknown* | Presently unavailable* |
| FGFR2b | PD-L1+ | Unknown† | Presently unavailable† |
| FGFR2 | HER2 (3+) | 0.3% | Su 201447 |
| FGFR2b | MSI/MMR | Unknown‡ | Presently unavailable‡ |
* A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (CLDN182) produced no articles.
† A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (PD-L1) produced five articles, none of which included information pertaining to the prevalence overlap of these two biomarkers.
‡ A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (MSI) produced seven articles, none of which included information pertaining to the prevalence overlap of these two biomarkers. A search using the search terms (gastric cancer) AND (FGFR2) AND (MMR) produced two articles, neither of which included information pertaining to the prevalence overlap of these two biomarkers.
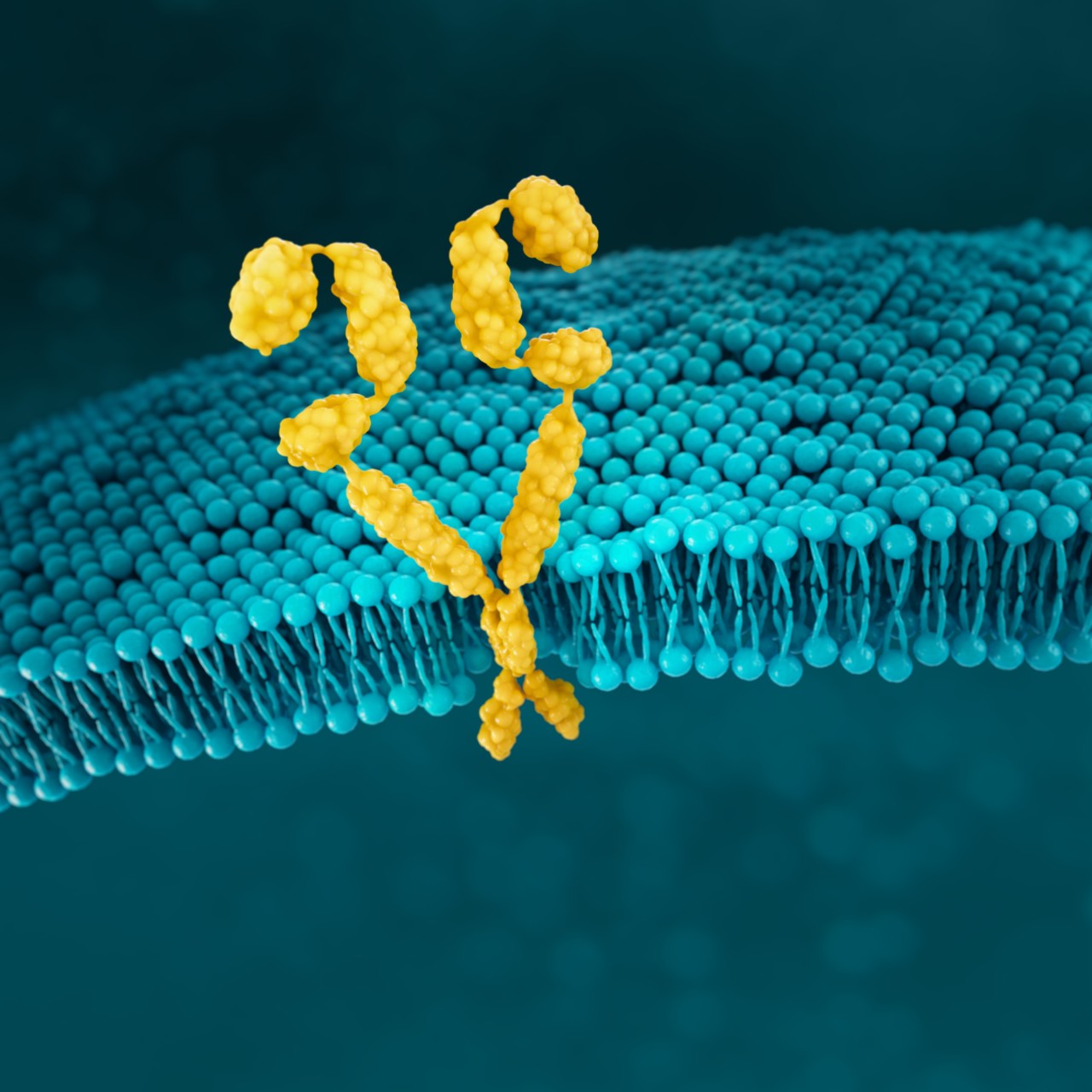
HER2
HER2 (human epidermal growth factor receptor 2) is a receptor-tyrosine kinase that is overexpressed and/or amplified in mG/GEJ cancer.9 HER2 expression is considered a prognostic factor in gastric cancer.48
HER2 is a proto-oncogene that is involved in signalling pathways, which leads to cell growth and differentiation.19
When HER2 is overexpressed, multiple HER2 protein receptors are formed and cell signalling is stronger, which results in enhanced responsiveness to growth factors and malignant growth.50
NORMAL CELL

Normal cell with low HER2 protein receptor expression and few HER2 receptor heterodimers at the cell surface.50
HER2+ CANCER CELL

HER2 is overexpressed and/or amplified, multiple HER2 receptor heterodimers are formed at the cell surface and cell signalling gets enhanced.50
HER2 positivity has been reported in 22% of advanced G/GEJ cancers.11
HER2 positivity: overexpression (IHC3+) and/or gene amplification (FISH-positive).
Detection of HER2 may be done with IHC, ISH methods and NGS, and is generally more associated with intestinal type tumours.11,17,19*
| Biomarker | Biomarker | Prevalence overlap | Reference |
|---|---|---|---|
| HER2 | CLDN18.2 | 12-15% | Pellino 2021,21 Pellino 2019,51 Baek 201952 |
| HER2 | PD-L1+ | 7.4% | Angell 201853 |
| HER2 | MSI | 11.1% | Angell 201853 |
| HER2 | FGFR2 | 0.3% | Su 201447 |
* IHC/ISH should be considered first, followed by additional NGS testing as appropriate.17
MSI
MSI expression is associated with genomic instability and increased susceptibility to tumour development.9
Microsatellites are repeated sequences of nucleotides in DNA.12
MICROSATELLITE STABLE TUMOUR
TUMOUR WITH HIGH MSI/MMRd
MMRd=MMR deficiency
In genomically stable tumours, with a functional MMR system, DNA replication errors occur rarely. Conversely, in the presence of high MSI/MMRd, DNA replication errors go undetected and unrepaired, leading to a tumour with a high mutational burden. Such hyper-mutated cancer cells excessively produce mutation-associated neoantigens, which are presented by MHC molecules on the cell surface to stimulate T-cell activation and tumour infiltration by immune cells. To counteract this vigorous immune response, tumour cells expose checkpoint molecules, e.g., PD-L1, to inhibit anti-tumour activity.56,57
MSI-H/MMRd expression has been reported in 4% of mG/GEJ cancers.26
MSI-H=MSI-high.
Detection of MSI/MMR is typically assessed with various methods.4
| Biomarker | Biomarker | Prevalence overlap | Reference |
|---|---|---|---|
| dMMR | CLDN18.2 | 15% | Pellino 202121 |
| MSI | PD-L1+ | 53.2% | Angell 201853 |
| MSI | HER2 (3+) | 4.2% | Angell 201853 |
| MSI/MMR | FGFR2b | Unknown* | Presently unavailable* |
*A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (MSI) produced seven articles, none of which included information pertaining to the prevalence overlap of these two biomarkers. A search using the search terms (gastric cancer) AND (FGFR2) AND (MMR) produced two articles, neither of which included information pertaining to the prevalence overlap of these two biomarkers.
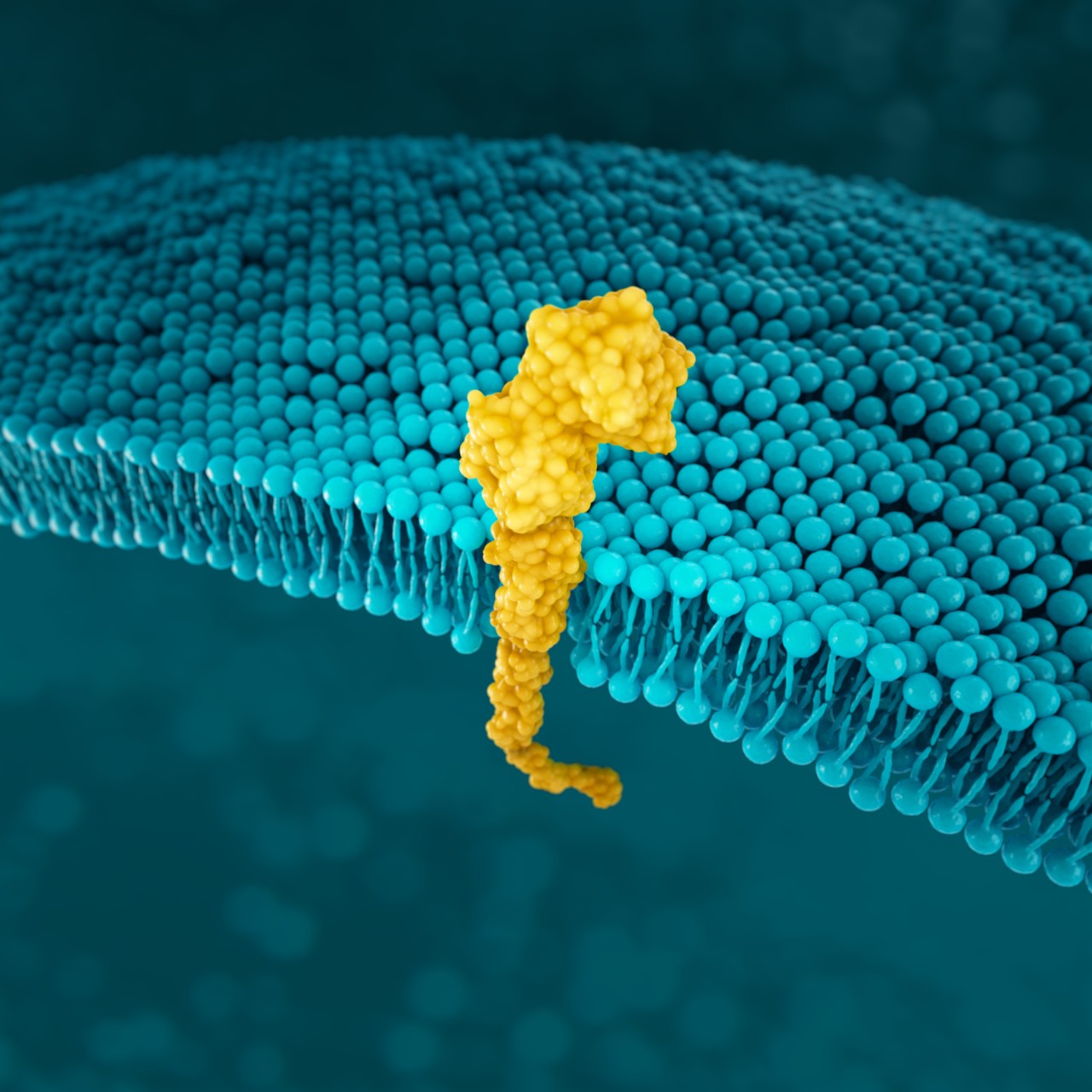
PD-L1
PD-L1 (programmed death-ligand 1) is a transmembrane protein that may be expressed on various tumour cells and/or immune cells.58
As the key regulator of immune tolerance and immune exhaustion, expression of PD-1 is tightly controlled: on naïve T-cells, PD-1 is only expressed in a low basal level; initial immune stimulation can induce PD-1 expression on T-cells, B-cells, macrophages and dendritic cells (see figure below). 60
NORMAL CELL

In normal tissues, PD-1/PD-L1 binding prevents an excessive immune response and protects tissue from damage through the induction of immune tolerance.60
GASTRIC CANCER CELL

In gastric cancer, CD274 focal amplification and IFN-γ-mediated signalling can lead to PD-L1 overexpression and T-cell exhaustion.60,61
Prevalence of PD-L1 has been reported for several positivity thresholds throughout clinical trials: 67-73% CPS ≥1, 29-31% CPS ≥5 and 16-18% CPS ≥1023,24*†






*Study population was limited to 592 patients with locally advanced or mG/GEJ cancer who experienced disease progression after first-line therapy with a platinum and fluoropyrimidine.23
†Study analysed 56 specimens from therapy-naïve biopsies from German patients with primarily non-metastatic gastric adenocarcinoma.24
| Biomarker | Biomarker | Prevalence overlap | Reference |
|---|---|---|---|
| PD-L1 (CPS ≥1) | CLDN18.2 | 28% | Pellino 202121 |
| PD-L1 (CPS ≥5 | CLDN18.2 | 20% | Pellino 202121 |
| PD-L1+ | HER2 (3+) | 3.2% | Angell 201853 |
| PD-L1+ | MSI | 53.2% | Angell 201853 |
| PD-L1 | FGFR2 | Unknown* | Presently unavailable* |
*A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (PD-L1) produced five articles, none of which included information pertaining to the prevalence overlap of these two biomarkers.
SUMMARY
The European Society for Medical Oncology (ESMO) guidelines on the diagnosis and treatment of gastric cancer recommend screening for HER2, PD-L1 and MSI-H/dMMR.18
In the US, guidelines for mG/GEJ cancer support the use of biomarkers to help map the path forward for patients:17
Biomarker testing provides more insight into mG/GEJ cancer as more biomarkers are being discovered:
*IHC/ISH should be considered first, followed by additional NGS testing as appropriate.17
As biomarker research continues, it expands our view of the patient population, reveals more information about the mG/GEJ cancer landscape, and helps inform clinical decisions.
Update your knowledge about biomarkers in gastric cancer
By signing up below, you agree to receive updates about emerging biomarkers in mG/GEJ cancer. Please note that fields marked with (*) are required.
You will now receive email updates about emerging biomarkers in mG/GEJ cancer, and
you may be contacted by an Astellas representative.
I hereby consent that Astellas Pharma Nordic*) can use the personal details I have provided for the purpose of sending me healthcare, medical and scientific-related information and materials, including invitations** for Astellas events and activities by email, which Astellas believes may be of value to me, relevant to my work or tailored to my professional profile and may be promotional in nature.
IMPORTANT: Your consent is voluntary and you can withdraw it at any time by clicking the “unsubscribe” link in any email communication you receive from Astellas going forward, or alternatively by sending an email to privacy@astellas.com
Astellas is committed to protecting your personal information and will only use it in accordance with the applicable data privacy law and our Astellas Privacy Notice. For further information on how we process and protect your personal information and how you may exercise your data protection rights, please refer to our Privacy Notice at http://policy.astellas.dk/en-privacy-notice-hcp/
If you have any questions or concerns, please contact us at privacy@astellas.com.
*) For the purpose of this consent, “Astellas Pharma Nordic” shall mean Astellas Pharma a/s (Denmark), Astellas Pharma AB (Sweden), Astellas Pharma (Norway) and Astellas Pharma A/S, filial in Finland (Finland).
** Astellas complies with the current Lif agreements in the Danish Regions, LMI Industry Rules in Norway and PIF regulations in Finland and will not send any invitations directly to any hospital-employed HCPs in Denmark and Norway or HCPs employed by the public healthcare services in Finland where not allowed.
REFERENCES
1. Casamayor M, Morlock R, Maeda H, Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience 2018;12:883. eCollection 2018.
2. Larønningen S, Ferlay J, Beydogan H, et al (2022). NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 9.2 (23.06.2022). Association of the Nordic Cancer Registries. Cancer Registry of Norway. Available from: https://nordcan.iarc.fr/en/dataviz/survival_table?sexes=2. Accessed 14-10-2022
3. Global Cancer Observatory, International Agency for Research on Cancer 2022. https://gco.iarc.fr/today/explore. Accessed 14-10-2022
4. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
5. Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 2008;14(23):7624-34.
6. Dottermusch M, Krüger S, Behrens HM, et al. Expression of the potential therapeutic target Claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch 2019;475(5):563-71.
7. Coati I, Lotz G, Fanelli GN, et al. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer 2019;121(3):257-63.
8. Li J, Zhang Y, Hu D, Gong T, Xu R, Gao J. Analysis of the expression and genetic alteration of CLDN18 in gastric cancer. Aging (Albany NY) 2020;12(14):14271-84.
9. Matsuoka T, Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J Gastroenterol 2018;24(26):2818-32.
10. Ahn S, Lee J, Hong M, et al. FGFR2 in gastric cancer: protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod Pathol 2016;29(9):1095-103.
11. Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015;18(3):476-84.
12. Baudrin LG, Deleuze JF, How-Kit A. Molecular and computational methods for the detection of microsatellite instability in cancer. Front Oncol 2018;8:621.
13. Kawazoe A, Shitara K, Kuboki Y, et al. Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein-Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer 2019;22(1):69-76.
14. Sahin U, Türeci Ö, Manikhas G, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol 2021;32(5):609-19.
15. Catenacci DV, Tesfaye A, Tejani M, et al. Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT Phase III study design. Future Oncol 2019;15(18):2073-82.
16. Jogo T, Nakamura Y, Shitara K, et al. Circulating tumor DNA analysis detects FGFR2 amplification and concurrent genomic alterations associated with FGFR inhibitor efficacy in advanced gastric cancer. Clin Cancer Res 2021;27(20):5619-27.
17. National Comprehensive Cancer NetworkR. NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesR): gastric cancer version 2.2022 (01-11-2022). https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed 14-10-2022.
18. Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022; 33(10):1005-1020.
19. Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol 2016;22(19):4619-25.
20. Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev 2015;41(10):868-76.
21. Pellino A, Brignola S, Riello E, et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J Pers Med 2021;11(11):1095.
22. Ooki A, Yamaguchi K. The beginning of the era of precision medicine for gastric cancer with fibroblast growth factor receptor 2 aberration. Gastric Cancer 2021;24(6):1169-83.
23 Fuchs CS, Ozgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated PD-L1-KEYNOTE-061 trial. Gastric Cancer 2022;25(1):197–206.
24. Schoemig-Markiefka B, Eschbach J, Scheel AH, et al. Optimized PD-L1 scoring of gastric cancer. Gastric Cancer 2021;24(5):1115-22.
25. Shitara K, Van Cutsem E, Bang Y-J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 2020;6(10):1571–80.
26. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4(5):e180013. Erratum in: JAMA Oncol 2019;5(4):579.
27. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398(10294):27-40.
28. Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 1984;312(5994):513-6.
29. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260(5109):816-9.
30. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192(7):1027-34.
31. Force J, Howie LJ, Abbott SE, et al. Early stage HER2-positive breast cancers not achieving a pCR from neoadjuvant trastuzumab- or pertuzumab-based regimens have an immunosuppressive phenotype. Clin Breast Cancer 2018;18(5):410–17.
32. Merok MA, Ahlquist T, Royrvik EC, et al. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol 2013; 24(5):1274–82.
33. Hashimoto I, Oshima T. Claudins and gastric cancer: An overview. Cancers 2022;14(2):290.
34. Niimi T, Nagashima K, Ward JM, et al. Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol 2001;21(21):7380-90.
35. Matsunobu T, Ishiwata T, Yoshino M, et al. Expression of keratinocyte growth factor receptor correlates with expansive growth and early stage of gastric cancer. Int J Oncol 2006;28(2):307-14
36. Smith SM, Wachter K, Horward A, et al. Clinical cancera 2021: ASCO’s report on progress against cancer. J Clin Oncol 2021;39(10):1165–84.
37. Arnold A, Daum S, von Winterfeld M, et al. Prognostic impact of Claudin 18.2 in gastric and esophageal adenocarcinomas. Clin Transl Oncol 2020;22(12):2357-63.
38. Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Türeci Ö. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol 2019;49(9):870-6.
39. Türeci O, Sahin U, Schulze-Bergkamen H, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol 2019;30(9):1487-95.
40. Bickenbach K, Strong VE. Comparisons of gastric cancer treatments: east vs. west. J Gastric Cancer 2012;12(4):262.
41. Lee JY, Gong EJ, Chung EJ, et al. The characteristics and prognosis of diffuse-type early gastric cancer diagnosed during health check-Ups. Gut Liver 2017;11(6):807–12.
42. Kurban G, Ishiwata T, Kudo M, Yokoyama M, Sugisaki Y, Naito Z. Expression of keratinocyte growth factor receptor (KGFR/FGFR2 IIIb) in human uterine cervical cancer. Oncol Rep 2004;11(5):987-91.
43. Katoh M. Cancer genomics and genetics of FGFR2 (Review). Int J Oncol 2008;33:233-237.
44. Azoury SC, Reddy S, Shukla V, Deng CX. Fibroblast growth factor receptor 2 (FGFR2) mutation related syndromic craniosynostosis. Int J Biol Sci 2017;13(12):1479-88.
45. Kim HS, Kim JH, Jang HJ, et al. Pathological and prognostic impacts of FGFR2 overexpression in gastric cancer: A meta-analysis. J Cancer 2019;10(1):20–7.
46. Hierro C, Alsina M, Sanchez M, et al. Targeting the fibroblast growth factor receptor 2 in gastric cancer: promise or pitfall? Ann Oncol 2017;28(6):1207–16.
47. Su X, Zhan P, Gavine P R, et al. FGFR2 amplification has prognostic significance in gastric cancer: results from a large international multicentre study. Br J Cancer 2014;110:967–75.
48. Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19(9):1523–29.
49. Vicario R, Peg V, Morancho B, et al. Patterns of HER2 gene amplification and response to anti-HER2 therapies. PLoS One;10(6):e0129876.
50. Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol 2001;12(Suppl 1):S3–8.
51. Pellino A, Riello E, Nappo F, et al. Targeted therapies in metastatic gastric cancer: Current knowledge and future perspectives. World J Gastroenterol 2019 October 14; 25(38): 5773-5788
52. Baek JH, Park DJ, Kim GY, et al. Clinical Implications of Claudin18.2 Expression in Patients With Gastric Cancer. Anticancer Res 2019;39:6973-6979.
53. Angell H K, Lee J, Kim K-M, et al. PD-L1 and immune infiltrates are differentially expressed in distinct subgroups of gastric cancer. OncoImmunology 2019;8(2):1544442.
54. Kim JY, Shin NR, Kim A, et al. Microsatellite instability status in gastric cancer: a reappraisal of its clinical significance and relationship with mucin phenotypes. Korean J Pathol 2013;47(1):28-35.
55. Velho S, Fernandes MS, Leite M, et al. Causes and consequences of microsatellite instability in gastric carcinogenesis. World J Gastroenterol 2014;20(44):16433–42.
56. Ratti M, Lampis A, Hahne JC, et al. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cell Mol Life Sci 2018;75(22):4151–62.
57. Eso Y, Shimizu T, Takeda H, et al. Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol 2020;55(1):15–26.
58. Wu Y, Chen W, Xu ZP, Gu W. PD-L1 distribution and perspective for cancer immunotherapy-blockade, knockdown, or inhibition. Front Immunol 2019;10:2022.
59. Qing Y, Li Q, Ren T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther 2015;9:901-9.
60. Ghosh C, Luong G, Sun Y. A snapshot of the PD-1/PD-L1 pathway. J Cancer 2021;12(9):2735–46.
61. Nakano H, Saito M, Nakajima S, et al. PD-L1 overexpression in EBV-positive gastric cancer is caused by unique genomic or epigenomic mechanisms. Sci Reps 2021;11(1):1982.
62. Budczies J, Denkert C, Győrffy B, et al. Chromosome 9p copy number gains involving PD-L1 are associated with a specific proliferation and immune-modulating gene expression program active across major cancer types. BMC Med Genomics 2017;10(1):74.
63. Wang Y, Wang H, Yao H, et al. Regulation of PD-L1: Emerging routes for targeting tumor immune evasion. Front Pharmacol 2018;9:536.
64. Saito H, Kono Y, Murakami Y, et al. Highly activated PD-1/PD-L1 pathway in gastric gancer with PD-L1 expression. Anticancer Res 2018;38(1):107–12.